Clinical Study to Evaluate the Efficacy and Safety of a Hair Serum Product in Healthy Adult Male and Female Volunteers with Hair Fall
Study Design
An open-label study was conducted in 42 healthy male and female volunteers with hair fall to evaluate the efficacy and safety of a hair serum formulation.
Treatment
Hair serum is a hair growth formulation containing Saberry® (Emblica officinalis extract, standardized for 10% β-glucogallin), Cococin™ (freeze-dried Cocos nucifera water solids) and PeptiSeLect® (GGLSM) in the form of water-soluble powder as active ingredients. The formulation contained peanut shell extract to prevent allergic reactions, Sandalore® (sandalwood odorant) for aroma, Cosmoperine® (Tetrahydropiperine), a natural and safe bioavailability enhancer, and other accepted excipients. The volunteers used the hair serum daily for three months. TrichoScan® was used to measure hair growth rate and hair density. Dermatologists and a self-assessment questionnaire assessed hair thinning and hair fall reduction.
Results
Instrument assessment by TrichoScan®
Hair Serum treatment showed a significant improvement in hair density (p<0.0001), hair growth rate (p<0.0001), vellus hair density (p<0.0001), and terminal hair density (p<0.0001) compared to baseline.
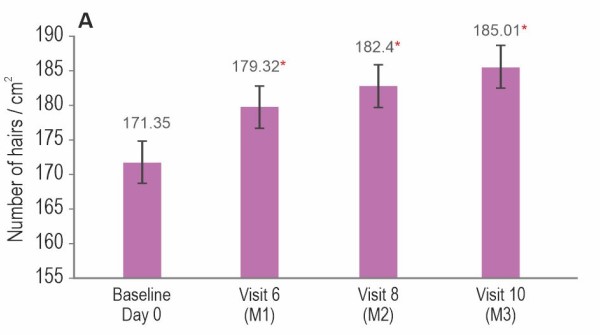
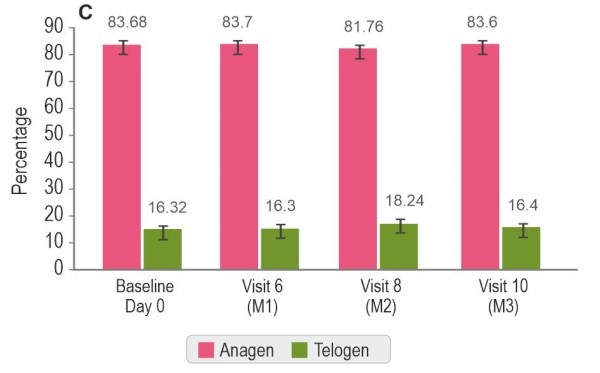


Values are expressed as mean±SE; *p<0.0001 by t-test
Dermatological assessment
Hair Serum treatment showed a significant reduction in hair fall with bulb (p<0.0001) and without bulb (p<0.0001) and hair thinning (p<0.0001).
Self-assessment questionnaire (SAQ)
Hair Serum treatment showed a significant improvement in overall hairfall rate, hair texture, hair volume and scalp itching compared to baseline.
No adverse events were reported during the study.
Conclusion
Clinical study showed that by applying novel hair serum formulation on healthy volunteers with moderate hair loss for three months could be a safe alternative to manage hair thinning and induce hair growth.
Continue Reading: doi: 10.2147/CCID.S271013